Buoyancy
When a body is immersed completely or partially in
a fluid, the fluid exerts an upward force on the body. This force is known as buoyancy.
The buoyancy exerted by a fluid on a substance
immersed in it will be equal to the loss of weight of the substance in that
fluid.
Factors affecting buoyancy
What are the factors which influence the buoyancy?
— Density of fluid
— Volume of the object immersed in the fluid
Archimedes principle
When an object is immersed partially or completely
in a fluid, the buoyancy experienced by it will be equal to the weight of the
fluid displaced by it. This principle was put forward by Archimedes and it is
known as Archimedes principle.
Principle of floatation
Weight of a floating body is equal to the weight of the fluid displaced by it. When a body is fully immersed in a fluid the volume of the displaced fluid will be equal to the volume of the body.
Relative density
Relative density of a substance is the ratio of the density of the
substance to the density of water.
Relative density = 𝑑𝑒𝑛𝑠𝑖𝑡𝑦 𝑜𝑓 𝑠𝑢𝑏𝑠𝑡𝑎𝑐𝑒 /𝑑𝑒𝑛𝑠𝑖𝑡𝑦 𝑜𝑓 𝑤a𝑡𝑒𝑟
Since it is a ratio relative density has no units
Which device is used to measure the relative density of a
liquid?
Ans: Hydrometer
Which device is used to test the purity of milk?
Ans: Lactometer
Lactometer is basically a hydrometer.
Pascal's law
The pressure applied at any point of a liquid at
rest in a closed system will be experienced equally at all parts of the liquid.
This is known as Pascal’s law.
Volume of a liquid cannot be changed using pressure. This is the basis of Pascal’s law.
Name some devices which work on the basis of Pascal’s law?
Hydraulic brake of vehicles, hydraulic jack,
hydraulic press, hydraulic lift etc.
Explain the working principle of hydraulic jack?
In a hydraulic jack two cylinders of different
cross sectional areas are connected using a pipe and filled with water. Let a
force F1 is applied on the water filled in the cylinder of small
cross sectional area using a piston with area of cross section A1.
Then the same pressure will be experienced on the piston on the water filled in
the cylinder of large cross sectional area according to Pascal’s law.
Let this force be F2
We know P = 𝐹/𝐴
F1/A1 = F2/A2
F2 = (A2 /A1) x F1
The force experienced on large piston is
proportional to its area. If the area of the large piston is 100 times that of
the small piston, the force experienced on the large piston will be 100 times
the force applied on the small piston.
Ans:
Capillarity
The rise or depression of a liquid in a narrow tube or a minute hole is capillarity.
example: in a kerosene lamp kerosene rises up through wick, in rainy season dampness spreads on walls, a piece of chalk is used to blot ink.
Give example for a liquid in which capillary
depression occurs?
What is surface tension?
Due to the mutual attraction of the molecules on
the surface of a liquid, the surface is stretched like a membrane. The force
responsible for this is called surface tension.
FORCE BETWEEN MOLECULES
¢ Cohesive Force
The attraction between the molecules of the same type is called cohesive force. The surface tension is due to the cohesive
force between the molecule on the
surface of the liquid.
¢ Adhesive Force
The attraction between the molecules of different types of substances is called adhesive force.
When does capillary rise occur?
Ans:
Capillary rise occurs when the adhesive force is greater than the cohesive force. Water rises up in a small glass tube because the adhesive force between water molecules and glass molecules is greater than the cohesive force between water molecules.
When does capillary depression occur?
Ans:
Capillary depression occurs when the cohesive
force is greater than the adhesive force. Mercury depresses down in a small glass tube because
the cohesive force between mercury molecules is greater than the adhesive force
between mercury molecules and glass molecules.
When the diameter of the capillary tube decreases
the capillary rise increases
Viscous force
Give examples for liquids having greater viscosity and lower viscosity than water.
Liquids of greater viscosity are the viscous
liquids.
Liquids of lower viscosity are called mobile
liquids.
When temperature increases viscosity of a liquid
decreases.
A person who had an electric shock is to be
massaged. Why?
Ans:
Let us assess
1) The wait of a piece of stone in air is 120N and
its wait in water is 100N. Calculate the buoyancy experienced by the stone?
Ans:
Loss of weight in water = 120N – 100N
=
20N
Buoyancy experienced by the stone= 20N
2) A body which floated in water sank when put in
kerosene. Why did it happen?
Ans:
Density of the object is lesser than that of
water. So it floats on water. The density of the object is greater than that of
the kerosene. So the body sinks in kerosene.
a). When it is in the liquid A, buoyancy is greater than the gravitational
force. In liquid B, gravitational force is greater than the buoyancy.
b) Liquid A is denser than the object. The body floats on the liquid because
it is lighter than the liquid.
Ans:
a)Weight of the body in water = 1000N – 250N
=
750N
b) Weight in water = 0N
Weight of the water displaced = 1000N
Ans:
a). Chalk has many pores in it. When it is placed over ink, due to capillarity
the ink will be absorbed by the small pores in chalk.
b)Tissue paper is porous. Therefore it can absorb sweat due to capillarity of
the pores in the tissue paper.
Ans:
Correct figure is A.
The liquid in the test tube undergo capillary
depression. Since the cohesive force between the liquid molecules is greater
than the adhesive force between the liquid molecules and glass molecules the
liquid undergo depression at the walls of the test tube and glass tube.



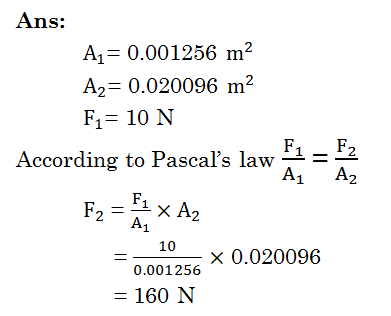








.png)

.png)

.png)

.jpg)
.jpg)
.png)






0 Comments